ES2B-C001
– a Her2-VLP-based vaccine in development for human breast cancer therapy
Overview
Target: HER2 extracellular domainMode of action: HER2 antagonist
Target population
HER2-expressing cancers, in first instance HER2+ breast cancerWe aim to elicit a broader immune response than HER2 epitope based vaccines, by targeting the entire HER2 extracellular domain (ECD)
Status
Why are we targeting HER-2?
The HER2 (human epidermal growth factor receptor 2) is a protein that plays a crucial role in the growth, division, and repair of cells. In context of cancer, Her2 is overexpressed or amplified in certain types of cancer, most notably breast cancer. This overexpression leads to increased signalling for cell growth and progression of cancer. In cases of human breast cancer, about 20-25% of patients are classified as HER2-positive. These cancers are more aggressive and have higher likelihood of recurrence compared to HER2-negative cancers.
Results to date
Vaccines are promising therapeutic alternatives to monoclonal antibodies against HER2 breast cancer. ES2B-C001 has shown promising results in preclinical tests. Using a virus-like particle (VLP) approach to deliver HER2 ECD, the ES2B-C001 vaccine triggered a strong immune response and effectively combated tumour growth and the spread of cancer in mouse models of HER2 positive breast cancer. The vaccine induced a durable polyclonal antibody response, which continued for several months after the final vaccination. The sera from vaccinated animals were effective in blocking the growth of breast cancer cells in vitro, including cells that were resistant to trastuzumab, a common breast cancer treatment.
Prevention of mammary carcinoma
Vaccination with only two doses of adjuvanted ES2B-C001 completely prevented onset of mammary carcinoma in human HER2 transgenic Delta 16 mice
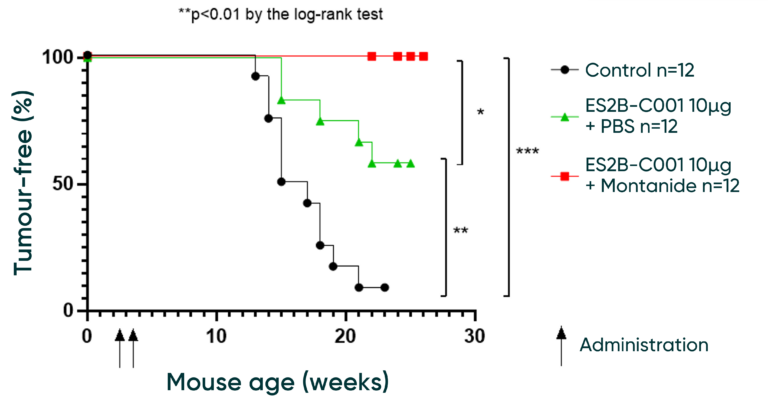
Kaplan-Meier survival curve demonstrated the efficacy of ES2B-C001 treatments in preventing tumours in mice. Both the ES2B-C001 10 µg and ES2B-C001 10µg + adjuvant groups show a higher percentage of tumour-free mice over time compared to the control group, indicating the potential effectiveness of these treatments (Figure below).
With Adjuvant: Mice receiving 10µg of ES2B-C001 plus adjuvant demonstrate a significantly higher tumour-free survival rate compared to both the control and non-adjuvant groups, maintaining 100% tumour-free status up to nearly 30 weeks of age.
Without Adjuvant: In comparison to the control group, mice injected with 10µg of ES2B-C001 alone show an increased percentage of tumour-free survival up to approximately 22 weeks of age, after which the survival rate declines. This suggests that ES2B-C001 alone can delay tumour development relative to the control.
These conclusions highlight the beneficial effect of the adjuvant in enhancing the anti-tumour efficacy of the ES2B-C001 µg injection. The annotations “*p<0.05” and “**p<0.01” by the log-rank test indicate statistical significance in the differences observed between groups at certain points on the curve. The ***p<0.001 is considered highly significant and implies that the observed results are very unlikely to have occurred by chance.
Source: Studies conducted by Alma Mater Institute on Healthy Planet & DIMES, University of Bologna.
Ref. Ruzzi, et al., Biomedicines 2022, 10, 2654
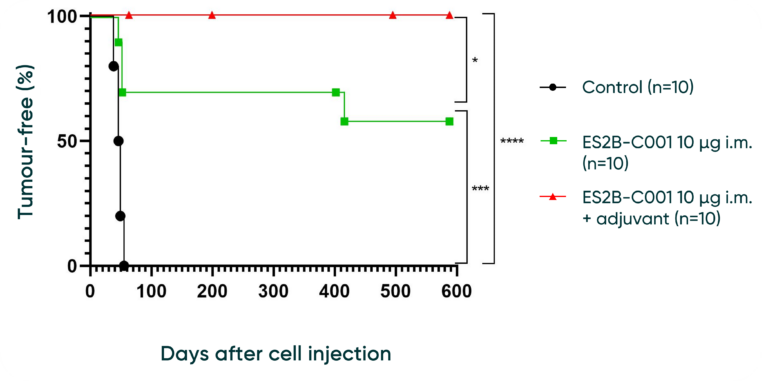
Therapeutic vaccination of FVB mice
Vaccinations completely inhibited cancer cells growth and resulted in 100% survival until at least 600 days
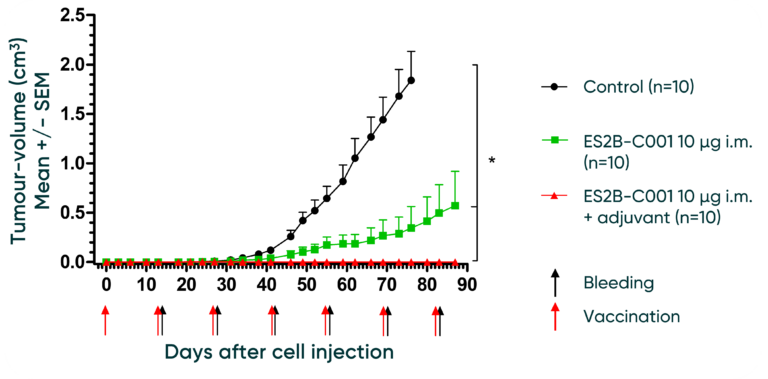
These conclusions indicate that while the 10 µg of ES2B-C001 by i.m. injection shows some effectiveness in controlling tumour growth compared to the control. The addition of adjuvant significantly enhances this effect. Further research would be beneficial to validate these results and explore the potential for clinical applications.
With Adjuvant: When adjuvant is added to the ES2B-C001 and administered by i.m. injection (represented by the red line with cross markers), the tumour growth rate is completely inhibited compared to the control group. The tumour volumes remain consistently lower than the control group throughout the observed period of 80 days, suggesting a substantial improvement in controlling tumour growth with the addition of adjuvant.
Without Adjuvant: Compared to the control group (represented by the black line with diamond markers), the mice injected with ES2B-C001 (represented by the green line with star markers) show a slower rate of tumour growth, suggesting effectiveness of the ES2B-C001 injection in controlling tumour growth.
With Adjuvant: Mice injected with ES2B-C001 + adjuvant demonstrate a higher efficacy, maintaining 100% tumour-free status throughout the entire duration of observation up to day 600. This significantly outperforms the control group.
Without Adjuvant: Mice injected with ES2B-C001 without adjuvant resulted in 60% tumour-free status.
Source: Studies conducted by Alma Mater Institute on Healthy Planet & DIMES, University of Bologna.
Ref. Ruzzi, et al., Biomedicines 2022, 10, 2654
